REVIEW ARTICLE
Exploring the Role of Mesenchymal Stem Cells During Normothermic Organ Perfusion: A New Paradigm to Enhance Outcome Following Allograft Transplantation
Mohamed Morsy1, *, Mohammad Ayaz Hossain2, Atul Bagul3
Article Information
Identifiers and Pagination:
Year: 2018Volume: 5
First Page: 47
Last Page: 52
Publisher Id: TOSCJ-5-47
DOI: 10.2174/1876893801805010047
Article History:
Received Date: 27/6/2018Revision Received Date: 19/10/2018
Acceptance Date: 2/11/2018
Electronic publication date: 30/11/2018
Collection year: 2018
open-access license: This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International Public License (CC-BY 4.0), a copy of which is available at: https://creativecommons.org/licenses/by/4.0/legalcode. This license permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Background:
Normothermic Machine Perfusion (NMP) has been established in the field of solid organ transplantation for both liver and kidney allografts. The ability to perfuse organs at body temperature enables viability assessment as well as optimisation prior to implantation.
Discussion:
A recent in vitro report of the use of Mesenchymal Stem Cells (MSCs) in the use of a normothermic lung perfusion circuit has raised the possibility of their use in solid organ transplantation. The aim of this short review is to outline the potential uses of bone marrow derived MSCs for their use in renal allograft ex vivo NMP. An overview is provided of current literature of NMP as well as theorised uses for MSCs.
1. INTRODUCTION
Mesenchymal Stem Cells (MSCs) of bone marrow origin are a multipotent progenitor cells [1]. They express intermediate Class 1 MHC and express CD105, CD73 and CD90 while lack expression of both haematopiotic and endothelial lineage markers [2]. They have immunomodulatory and immune-evasive properties, making them suitable for cell based therapy [3]. These properties lend them to variable applications in the field of organ transplantation. Such applications include immunomodulation [4], post ischaemia reperfusion [5, 6] and more recently in the field of lung transplantation, during Extracorporeal Machine Perfusion (EMP) [7].
Kidney transplantation remains the treatment of choice for End Stage Renal Disease (ESRD). Optimisation of renal transplant outcomes has been explored with recent developments in Normothermic Machine Perfusion (NMP). Monitoring and imaging of systemically delivered MSCs present a challenge in efficient cell seeding [8]. A closed ex-vivo NMP circuit therefore lends itself to overcome some of these challenges.
To date there is little in the way of clinical trials related to the use of MSCs in NMP kidney transplants.
This review provides an overview of potential applications of MSCs with NMP whilst theorising outcomes given the preclinical in vitro evidence to date.
1.1. Normothermic Machine Perfusion
Since the 1960s, static cold storage (a cold organ in a box of ice) has been the mainstay in preservation. The worldwide shortage of organs has driven research in to the use of machine perfusion pre-implantation to both assess as well as optimise function. Machine Perfusion (MP) can either be cold or warm. Warm perfusion, usually at body temperature, is termed normothermic (NMP). Several machines for liver, kidneys, lungs and hearts exist in the clinical practise [9]. Continuous perfusion of the organ at body temperature ex-vivo, permits efficient oxygen delivery as well as essential nutrients in a closed circuit. The perfusate is renewed or filtered and in the case of renal ex-vivo NMP, the production of urine observed can be collected for analysis [10]. Real time assessment of the organ during NMP can be made with clinical and biochemical parameters studied in order to “risk stratify” to outcome. Compared to static cold storage, a more sophisticated assessment can be made.
NMP devices have aided the expansion of Donation after Circulatory Death (DCD) organs that, due to the exposure of prolonged warm ischaemia, have conventionally been considered inferior to both living and brain dead donors [11]. The ability to machine perfuse under artificial physiological parameters, enables organ assessment prior to implantation.
Renal transplantation remains the treatment of choice for patients with End Stage Renal Disease (ESRD). DCD renal allografts have higher rates of Delayed Graft Function (DGF) as well as Primary Non Function (PNF) [11]. Early study into the use of ex-vivo NMP has been encouraging with significantly lower rates of DGF observed in machine perfused kidney allografts versus static cold storage [10]. Long term survival outcomes from graft rejection as well as chronic allograft failure in the form of interstitial fibrosis lend the use of NMP towards stem cell therapy. To date there are no combined studies into the effect of renal allograft NMP and stem cell usage.
1.2. Mesenchymal Stem cells and Immunomodulation
Mesenchymal stem cells are multipotent progenitors, which in current clinical practise are usually derived from Bone Marrow (BM). They have immunomodulatory and immune-evasive properties, making them suitable for cell based therapy. MSCs are known to activate T and B regulatory cells and interfere with maturation and activation of Antigen Presenting Cells (APCs) [2, 12, 13]. The potential use of MSCs is not solely limited to cellular repair and regeneration. Preclinical studies elucidated their immunoregulatory potential; however their clinical use still remains to be explored. Accumulated evidence showed MSC helps the development of tolerance through the induction of T and B regulatory cells and its inhibitory and modulatory effect on various immune effector cells including APCs, macrophages, neutrophils, mast cells, T and B cells also enhance the anti-inflammatory pathways and supress the cytotoxic activities of NK cells [2, 12, 13]. They produce wide range of Th2, anti-inflammatory [14] and ant apoptotic [15] effectors including TGF-B, HGF, NO, HO-1, IDO, TNF-TRAIL and PD-L1.
Preclinical studies established their safety and their effect on enhancing graft survival, diminishing rejection rate and lymphocyte proliferation [16-18]. However, early clinical study reports didn’t show similar potential yet due to various reasons including their use in conjunction with immunosuppression medications that already supress various function, the influence of microenvironments and the immunogenicity when allogenic - MSC used [18-21].
A recent study by Pan et al, demonstrated similar 2 year survival outcomes of patients given low dose Calcineurin Inhibitor (CNI) with donor derived BM-MSCs compared with standard CNI use alone [4]. The authors do not give a formal explanation for the mechanism of action and the method of MSC immunosuppression is not completely understood. Early in vitro work has suggested that autologous T-cell proliferation is inhibited by MSCs through the secretion of both transforming growth factor-β [22]. Indeed some authors have suggested that because of the production of extracellular vesicles, such as exosomes, as well as cytokines and growth factors, MSC immunomodulation is related to the trophic and stimulatory function over the cellular differentiation aspect [23]. Both low levels of Major Histocompatibility Complex (MHC) class I & II as well as CD-40 expression further support this hypothesis [23]. This is, however, contradictory to previously held belief that BM-MSCs do not express MHC class II or their co-stimulatory molecules including CD-40 [24].
1.3. Ischaemia Reperfusion
Ischaemia Reperfusion Injury (IRI), is the development of organ dysfunction following arterial clamp release and subsequent perfusion of the allograft. Reactive oxygen species’ (ROS) development at the time of IRI is a leading contributor DCD renal allograft dysfunction [25]. The role of MSCs in ameliorating IRI, remains unclear. Casiraghi et al have studied the topic in-vivo and in-vitro. The group postulated that the presence of MSCs engraft during the reperfusion process, induces complement activation as well as expression of pro-inflammatory cytokines, which contribute to the subsequent organ dysfunction [26]. They hypothesized that MSCs could be directed towards acquisition of pro-inflammatory functions when exposed to specific mediators of inflammation such as ROS during renal IRI. This non NMP work, however, contrasts that found in the only clinical study of note in MSC use with an NMP circuit.
Both vascular endothelial growth factor and IL-8 are key mediators in IRI. In their ex-vivo NMP porcine lung model which perfused human-umbilical cord derived MSCs, Mordant and associates, observed elevated VEGF concentrations in the lung perfusate as well as suppression of IL-8 [7]. It may be possible that with the use of both a ROS species and lymphocyte scavenger within the circuit, the pro-inflammatory induction of MSCs theorised by Caseraghi’s group is potentially overcome, therefore permitting favourable cellular expression of VEGF. Mordants work supports the use of ex-vivo NMP to pre condition the allograft for MSC acceptance by creating an environment for beneficial cellular MSC expression. The groups’ machine, however, is still at its infancy.
1.4. Outcome Measures of MSCs in NMP
Delivery of MSCs in Mordants’ group was either endotracheally or intra-arterially in 18 animals [7]. After a cold storage period of 18 hours, ex-vivo lung perfusion (EVLP) was sustained for 12 hours with administration of human umbilical cord MSCs after one hour. One of the main observations of the model was the retention of the cells during the first few minutes after retention. Measurement of CD73+ cells as biomarkers of MSCs concentration, revealed high perfusate retention (83%) after the first 2 minutes followed by 100% depletion by 30 minutes. The converse elevated concentrations of CD73+ in the leucocyte scavenger of the machine highlighted a critical limitation. Intravascular administration albeit more effective than endobronchial, produce high losses through the scavenger mechanism. The resultant demonstration of human DNA in the lung parenchyma after 12 hours by the group using PCR, confirms the action of the MSCs. The aforementioned expression of IL-8 in the perfusate validated the function of the MSCs during this period. A clear limitation of the NMP model reported is the duration of the CS time along with the machine perfusion. Eighteen hours of cold storage followed by 12 hours of NMP would be considered extreme for solid organ use. In lung transplantation, current clinical practise is to ex vivo perfuse for upto four hours. Even though the authors’ have not commented, with further optimization, it may be possible that MSCs infusion of the circuit would enable longer perfusion and storage times in order to establish VEGF expression and in turn initiate IR repair mechanisms prior to implantation. It is not clear from this model or report alone, the dose effect relationship of the delivered human umbilcal cord MSCs on IR and inflammatory repair on immunomodulation. A potential target for renal allograft NMP would be to examine the expression of interleukin factors within the NMP circuit at time intervals as well and stratify this against outcome in an experimental large animal model.
1.5. Future Proposals
Preclinical studies confirmed the safe use of MSC with promising results that demonstrated the immunomodulatory potential of MSC and their possible role in anti-rejection and immunosuppression maintenance protocols in organ transplantation. However, early clinical studies struggled to show this optimism. This was explained by various reasons including the in vitro microenvironment, type and donor age, Isolation techniques and site of isolation [27]. A surge of trials is underway tackling these challenges including proposals [27] utilising genetic modifications and targeted delivery systems with P-Selectin Glycoprotien Ligand-1 [28] or Etanercept [29]. We propose a novel technique in using MSC during ex-vivo normothermic kidney perfusion utilising the plasticity of MSC in organ optimisation and minimising the effect of reperfusion injury through their anti-apoptotic and anti-inflammatory functions.
CONCLUSION
There are no reports demonstrating the use of BM-MSCs during an ex vivo NMP of renal allografts either clinically or experimentally. As such, we have reviewed one study utilising human umbilical cord isolated MSCs to determine the potential challenges facing future use in solid organ transplantation. The benefit of MSC use in a closed circuit is the ability of the cell clone to home to the organ and differentiate providing potential immunomodulation as well an IR inflammation repair post reperfusion. However, delivery of the MSC within the circuit has been shown to have some limitations as summarised in Fig. (1). The dose optimisation of the MSC clone will be a priority in retention of the cells within the circuit. Confirmation of homing will need to be demonstrated through expression markers, although this process has been difficult using conventional imaging [8]. Collection of urinary and venous sampling will also determine percentage uptake, although this is only a theory at present as there are no current experimental data to prove MSC or marker positivity in these samples from an ex vivo circuit. Nonetheless, the use of cells taken from the leucocyte scavenger in the circuit may determine the retention of the MSC population. Regular sampling from the scavenger may determine the necessary duration of perfusion. Mordants group acknowledges that 18 hours of cold ischaemia with 12 hours of warm perfusion is beyond the clinical application of an NMP circuit [7]. This period should be considerably shorter to align with current NMP kidney models and it is unclear whether 2 to 4 hours of perfusion would satisfy an adequate MSC effect.
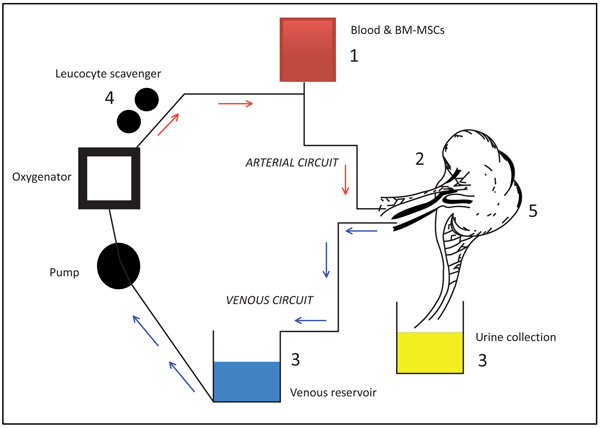 |
Fig. (1). Considerations for the use of MSCs in NMP. |
We acknowledge the limitations of this short review, whilst highlighting the need for further in vitro experimental reports of BM-MSCs in NMP circuits. Challenges have been demonstrated on the paucity of current literature and we look forward to reporting on future studies.
Schematic representation of a conventional renal allograft ex-vivo NMP circuit. Sites of potential experimental considerations for MSCs are listed as 1. MSC dose delivery optimisation, 2. Ensuring retention of MSCs during the period of NMP as well as monitoring homing, 3. Collection of samples from venous and urine effluent to assay expression markers e.g interleukins and Vascular Endothelial Growth Factor (VEGF), CD+ve cells, 4. Limiting loss of MSCs in the leucocyte scavenger and or utilizing the scavenger to assess retention 5. Histological efficacy of MSC cellular differentiation within the parenchyma.
CONSENT FOR PUBLICATION
Not applicable.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.




