RESEARCH ARTICLE
The Secretome of Mesenchymal Stem Cells Prevents Islet Beta Cell Apoptosis via an IL-10-Dependent Mechanism
Buthainah Al-Azzawi1, 3, Declan H. McGuigan2, Fiona N. Manderson Koivula2, Ajile Elttayef1, 3, Tina P. Dale1, Ying Yang1, Catriona Kelly2, Nicholas R. Forsyth1, *
Article Information
Identifiers and Pagination:
Year: 2020Volume: 6
First Page: 1
Last Page: 12
Publisher Id: TOSCJ-6-1
DOI: 10.2174/1876893802006010001
Article History:
Received Date: 30/12/2019Revision Received Date: 20/01/2020
Acceptance Date: 09/02/2020
Electronic publication date: 20/03/2020
Collection year: 2020
open-access license: This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International Public License (CC-BY 4.0), a copy of which is available at: https://creativecommons.org/licenses/by/4.0/legalcode. This license permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Background:
Type 1 Diabetes Mellitus (T1DM) is partly driven by autoimmune destruction of the pancreatic beta cell, facilitated by the release of inflammatory cytokines, including IFN-γ, TNF-α and IL-1β by cells of the innate immune system. Mesenchymal Stem Cells (MSCs) have been used to counteract autoimmunity in a range of therapeutic settings due to their secretion of trophic and immunomodulatory factors that ameliorate disease independently of the cells themselves.
Objective:
The aim of this study was to assess the effect of the secretome of human bone-marrow derived MSCs on cytokine-driven beta cell apoptosis.
Methods:
All experiments were conducted in two insulin-secreting islet cell lines (BRIN-BD11 and βTC1.6) with selected experiments confirmed in primary islets. MSC secretome was generated by conditioning serum-free media (MSC-CM) for 24 hours on sub-confluent MSC populations. The media was then removed and filtered in readiness for use.
Results:
Exposure to IFN-γ, TNF-α and IL-1β induced apoptosis in cell lines and primary islets. The addition of MSC-CM to cell lines and primary islets partially reversed cytokine-driven apoptosis. MSC-CM also restored glucose-stimulated insulin secretion in cytokine-treated cell lines, which was linked to improved cell viability following from cytokine challenge. Characterization of MSC-CM revealed significant concentrations of IL-4, IL-10, PIGF and VEGF. Of these, IL-10 alone prevented cytokine-driven apoptosis. Furthermore, the inhibition of IL-10 through the addition of a blocking antibody reversed the anti-apoptotic effects of MSC-CM.
Conclusion:
Overall, the protective effects of MSC-CM on islet beta cell survival appear to be largely IL-10-dependent.
1. INTRODUCTION
T1DM is a complex autoimmune disease in which several inflammatory cells inflict a coordinated assault on the pancreatic islets of Langerhans and the insulin-producing beta cells therein, contributing to an absolute insulin requirement. Autoimmune beta cell destruction begins when autoantigens (i.e., GAD65) are released during the spontaneous turnover of beta cells. The antigens are processed by antigen presenting cells and presented to CD4+ TH1 cells, which secrete cytokines including Interferon (IFN)γ, Tumour Necrosis Factor (TNF)-α, TNF-β and Interleukin (IL)-2. IFN-γ causes macrophages to become cytotoxic and release substantial quantities of cytokines (including IFN-γ, TNF-α and IL-1β) leading to beta cell apoptosis [1]. It is believed that beta cell mass is reduced by 70-80% at the time of diagnosis of T1DM. Due to the absence of detectable beta cell necrosis and variable degrees of insulitis, it has been suggested that beta cell loss occurs slowly over years [2]. This is supported by the detection of insulin antibodies years before the appearance of clinical symptoms in susceptible individuals [3].
Mesenchymal stem/stromal cells / Medicinal Signaling Cells (MSCs) are multipotent cells that can be found in almost all adult organs and tissues and are characterized by their immunomodulatory abilities. In the context of diabetes research, MSCs have been used to counteract autoimmunity and enhance islet engraftment and survival [4, 5]. Despite the reported antidiabetogenic effects of MSCs [6], the mechanism of action remains poorly understood. This is partly due to the diverse range of effects that MSCs and their secreted products have on the surrounding environment. Emerging evidence suggests that the therapeutic utility of MSCs could be based primarily on their production of trophic and immunomodulatory factors. Indeed, the infusion of MSC conditioned media (MSC-CM) every 3 days relieved hyperglycemia in a rodent model of T2DM [7]. The animals showed enhanced concentrations of c-peptide and insulin, as well as improvements in glucose metabolism. The authors concluded that these improvements largely stemmed from the secretion of cytokines and growth factors by the MSCs. An earlier study revealed that trophic factors from MSCs aided islet survival and function after transplantation [8]. This study reported high concentrations of IL-6, vascular endothelial growth factor-A (VEGF-A), hepatocyte growth factor (HGF), and transforming growth factor (TGF)-β found in MSC-CM. MSCs secrete soluble factors that play multifactorial roles in the regulation of circulating inflammatory cells. For example, MSCs secrete TGF-β and IL-10, which block T-cell proliferation [9, 10], while soluble factors secreted by MSCs are also believed to alter the secretion profile of dendritic cells leading to increased production of anti-inflammatory cytokines including IL-10 and decreased production of inflammatory cytokines including IFN-γ [11].
It has been hypothesized that MSCs may offer protection against diabetes through paracrine actions to include cytoprotective, anti-inflammatory, and anti-apoptotic effects [12]. The current study sought to characterize the effect of the secretome of human bone-marrow derived MSCs on cytokine-driven beta cell apoptosis. Here, we report that the secretome of human MSCs protects beta cell lines and primary islets from cytokine-driven apoptosis through an IL-10 dependent mechanism.
2. METHODS
2.1. Beta Cell Models
All experiments were conducted in two beta cell lines to ensure that data was not skewed by the nuances of any individual cell line. BRIN-BD11 cells were purchased from ECACC General Cell Collection (ECACC 10033003) and cultured as previously described [13]. βTC1.6 cells were purchased from ATCC (ATCC CRL-11506, LGC Standards, UK) and cultured according to the supplier’s instructions. In brief, BRIN-BD11 cells were cultured in RMPI and βTC1.6 cells cultured in DMEM (4.5g/L glucose). Both culture media were supplemented with 10% fetal bovine serum (FBS; Lonza, UK) and 1% Penicillin-Streptomycin (Lonza). The cells were routinely passaged with 1x trypsin/EDTA (Lonza).
Where possible, experimental results were confirmed in primary islets isolated from CD1 mice aged 12-16 weeks and bred in-house. All procedures were conducted in accordance with the Animals Scientific Procedures Act 1986. Animals were euthanized under Schedule 1 methods and the pancreas excised and transferred to Hank’s Balanced Salt Solution (HBSS) transport buffer comprising 0.14 M NaCl, 0.005 M KCl, 0.001 M CaCl2, 0.0004 M MgSO4, 0.0005 M MgCl2, 0.0003 M Na2HPO4, 0.0004 M KH2PO4, 0.006 M Glucose, 0.004 M NaHCO3 and 10 mM Hepes. The pancreas was chopped, placed in collagenase P (0.5 mg/ml collagenase clostridium histolyticum, (Fisher, UK) in HBSS), and agitated at 37°C for 10 minutes followed by the addition of HBSS supplemented with 0.1% Bovine Serum Albumin (Sigma, UK) to neutralize enzymatic action. Pancreatic tissue was then centrifuged for 5 mins at 1000 rpm, the pellet washed three times in wash buffer (HBSS + 5% FBS), the homogenized tissue passed through a fine mesh filter, and the filtrate centrifuged for 5 mins at 1000 rpm. Pelleted islets were resuspended in RPMI media supplemented with 5% FBS and 1% Penicillin-Streptomycin and hand-picked using a fine glass pipette. The islets were maintained in an incubator at 37°C and 5% CO2 for 24 hours before experimentation.
2.2. Cytokine Stimulation of Beta Cell Models
Recombinant Tumor Necrosis Factor-α (TNF-α), Interferon Gamma (IFN-γ) and Interleukin-1β (IL-1β) were purchased from PeproTech, UK. Cell lines were seeded at 1 x 105 cells/cm2 and allowed to attach overnight. Islets were seeded at a density of 50 islets/cm2 and maintained in culture overnight. Cell lines and islets were then exposed to a range of IFN-γ, TNF-α and IL-1β concentrations (0.1 ng/ml - 1000 ng/ml) for 24 h. Determination of cytokine concentrations that induced an approximate reduction in cell viability of 50% was established with MTT (Sigma, UK) in the first instance and induction of apoptosis confirmed by TUNEL assay (TUNEL in situ direct DNA fragmentation kit (Abcam, UK)).
2.3. Human Bone Marrow-Derived Mesenchymal Stem Cells (MSCs)
Human Bone Marrow Mononuclear cells (hMNCs) were purchased from Lonza, UK and hMSCs isolated according to a previously published methodology [14]. Mononuclear cells were seeded at a density of 1 x 105 MNC/cm2. Culture vessels were pre-coated with 10 ng/ml of fibronectin (Sigma, UK) in PBS for one hour at room temperature. Seeded MNC cells were maintained in DMEM media supplemented with 5% FBS, 1% L-Glutamine (Lonza, UK), 1% Non-essential amino acid (Lonza, UK) and 1% Penicillin Streptomycin Amphotericin-B (Lonza, UK). After one week, a 50% media change was performed and cells incubated for a further week after which a 100% media change was performed. Routine media changes were performed twice weekly thereafter. The cells were passaged at 80-90% confluency as described [14].
hMSC multipotency determination was established through differentiation into osteogenic, adipogenic and chondrogenic cells using chemical induction with differentiation media as outlined in the Supplementary Fig. (S1).
2.4. Preparation of MSC-Conditioned Media (CM)
MSC conditioned medium (MSC-CM) was prepared by medium-cell contact with 70% confluent hMSCs for 24 hours. MSCs were washed once with 10 ml PBS and twice with 10 ml of serum free DMEM. Either 15 ml RPMI-1640 or DMEM media (Lonza, UK) was then left in contact with the MSCs for 24 hours after which the conditioned media was collected, centrifuged to remove any cell debris, filtered through 0.2 µm filter then stored at -80°C until required for experimental use.
2.5. Measurements of Cellular Viability And Apoptosis
MTT reagent 5 mg/ml (Sigma, UK) was mixed with RPMI1640 media, added to cells and incubated for 2 hours at 37°C. MTT solution was removed and DMSO was added to each well before incubation at 37°C for further 45 minutes. The absorption was measured with a micro-plate reader (Dynatech, MR5000 version 3.7) at a wavelength of 570 nm with a reference wavelength reading at 650 nm.
Following optimization of cytokine concentration, TUNEL (terminal deoxynucleotidyl transferase mediated deoxyuridine triphosphate nick end labelling) assay (TUNEL in situ direct DNA fragmentation kit (Abcam, UK)) was used to determine if reductions in cell viability observed with the MTT assay resulted from apoptosis. The TUNEL assay was performed following a modified version of the manufacturer’s protocol. The medium was first removed from the cells and islets (islets were gently centrifuged at 900 rpm prior to each step in the following protocol), washed once with PBS, and fixed with 95% methanol for 10 mins. Methanol was removed and the cells washed twice with washing buffer, and then re-suspended in staining solution comprising reaction buffer, TDT enzyme, FITC_dUTP and ddH2O. The cells were incubated at 37 °C for one hour and the staining solution removed. The cells were washed with rinse buffer twice after which DAPI (4,6-Diamidino-2-phenylindole) (Sigma, UK) was added for 30 mins at room temperature. Images of cell lines were acquired by a fluorescent microscope (Olympus Fluoview, Nikon Eclipse, Japan) while islets were visualised using a laser scanning confocal microscope (Olympus, Japan).
2.6. Glucose-Stimulated Insulin Secretion
To determine the effect of MSC-CM on insulin secretion from pancreatic beta cells, cell lines were seeded at a density of 1 x 105 cells/cm2 and allowed to attach overnight. Following this step, the cells were treated with pro-inflammatory cytokines (IFN-γ. TNF-α, IL-1β) with and without MSC-CM. Glucose solutions were prepared in 1x Hepes buffered saline (HBS comprising 10 mM Hepes, 145 mM NaCl, 5 mM KCl and 1 mM MgSO4) at three different concentrations (1.1 mM, 5.6 mM and 16.7 mM D-Glucose). After exposing the cells to cytokines for 24 hours, the medium was removed and the cells were washed twice with 1 ml HBS followed by the addition of 1.1 mM glucose solution for 40 mins. This was then removed and 1.1, 5.6 or 16.7 mM glucose solution added for further 20 mins after which the supernatant was removed and stored at -20°C until further analysis. The cells were lysed using 200 μl/well/24well plate RIPA buffer (Sigma) and transferred to fresh tubes, which were maintained on ice with regular vortexing for 20 mins. Lysates were centrifuged at full speed and 4 °C for 20 mins. The total protein present in the resulting supernatants was quantified using the Pierce BCA Protein Assay Kit (Thermo Scientific, UK) according to the manufacturer’s instructions. Insulin secretion into the supernatants was quantified using ALPCO ELISA kits (ALPCO, USA) according to the manufacturer’s instructions.
2.7. Characterization of Conditioned Media
Following the identification of candidates from a secretome screen of MSC-CM (Marwan and Forsyth, under review), ELISA was used to quantify the concentration of interleukin-4 (IL-4), interleukin-10 (IL-10), vascular endothelial growth factor (VEGF) and placental growth factor (PIGF) in our MSC-CM. ELISA development kits were purchased from PeproTech (UK) and assays were developed for each cytokine or growth factor according to the manufacturer’s instructions.
2.8. Statistical Analysis
Data are presented as mean ± minus standard deviation (SD) for a given number of observations (n) as indicated in the Figure legends. Groups of data were compared using two-tailed unpaired Student t-tests, or One-way ANOVA with posthoc test (Graphpad, PRISM software, USA), with significance being accepted if P<0.05.
3. RESULTS
3.1. MSC-CM Ameliorates Cytokine-Driven Apoptosis in Beta Cell Models
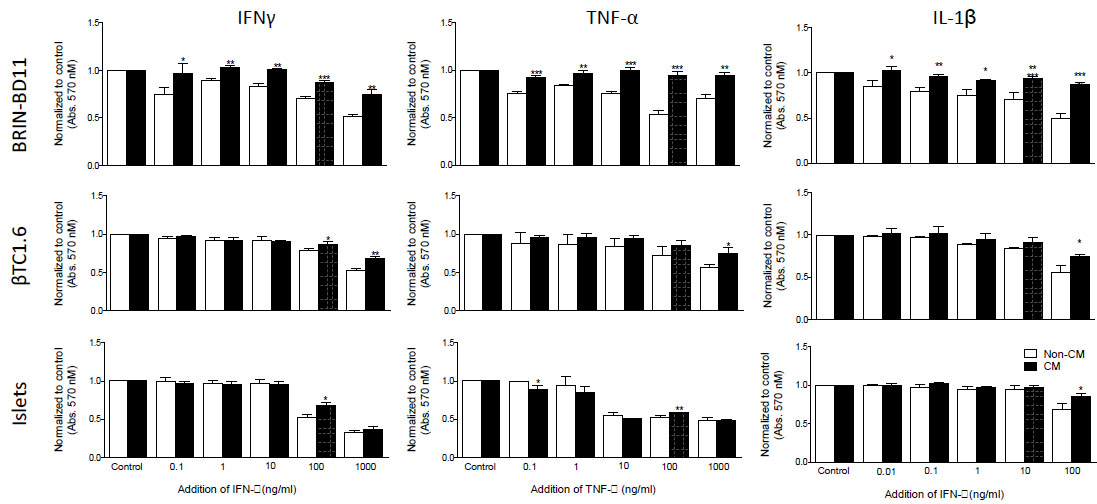
Cytokine concentration was optimized by MTT assay. Following 24h exposure to 1 µg/ml IFN-γ, 100 ng/ml TNF-α and 100 ng/ml IL-1β, both BRIN-BD11 and βTC1.6 cells displayed significant reductions in cellular viability of up to 50% (Fig. 1 and Supplementary Table S1). These concentrations were used for subsequent experiments. In primary islets, the equivalent concentrations were 100 ng/ml IFN-γ, 100 ng/ml TNF-α and 100 ng/ml IL-1β (Fig. 1 and Supplementary Table S1). In both the BRIN-BD11 and βTC1.6 cell lines, MSC-CM was able to ameliorate (P<0.05 – 0.001) these reductions in cellular viability (Fig. 1). Modest improvements (P<0.05) in viability were observed in primary islets in response to both IFN-γ and IL-1β in the presence of MSC-CM. However, MSC-CM had little effect on the viability of primary islets following exposure to TNF-α (Fig. 1).
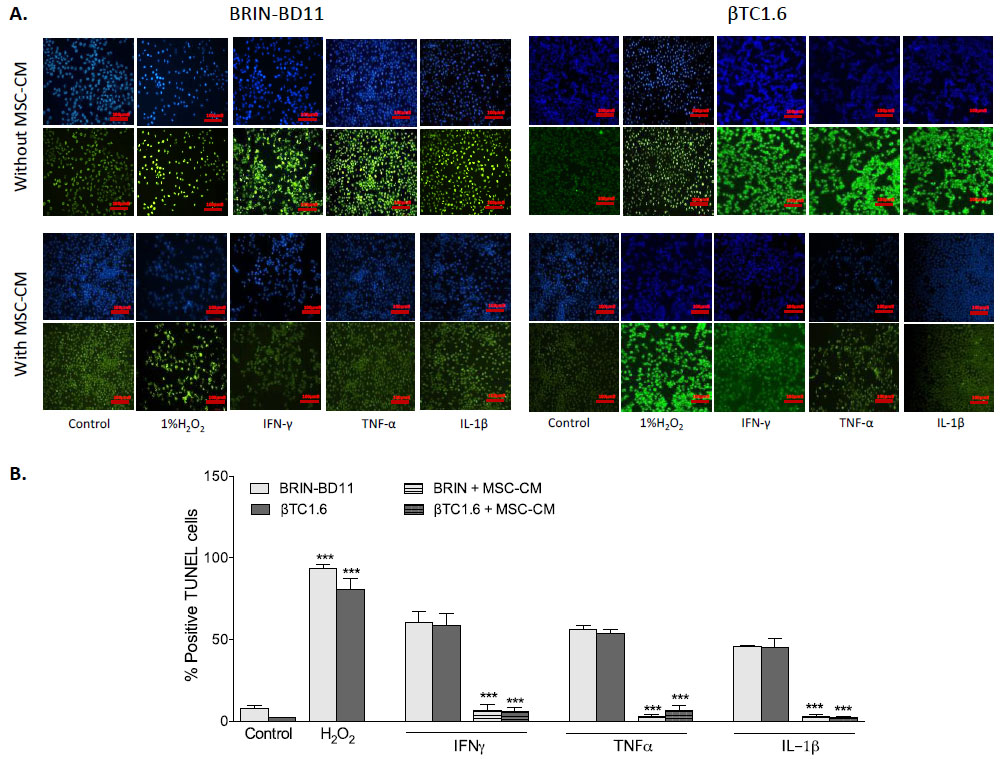
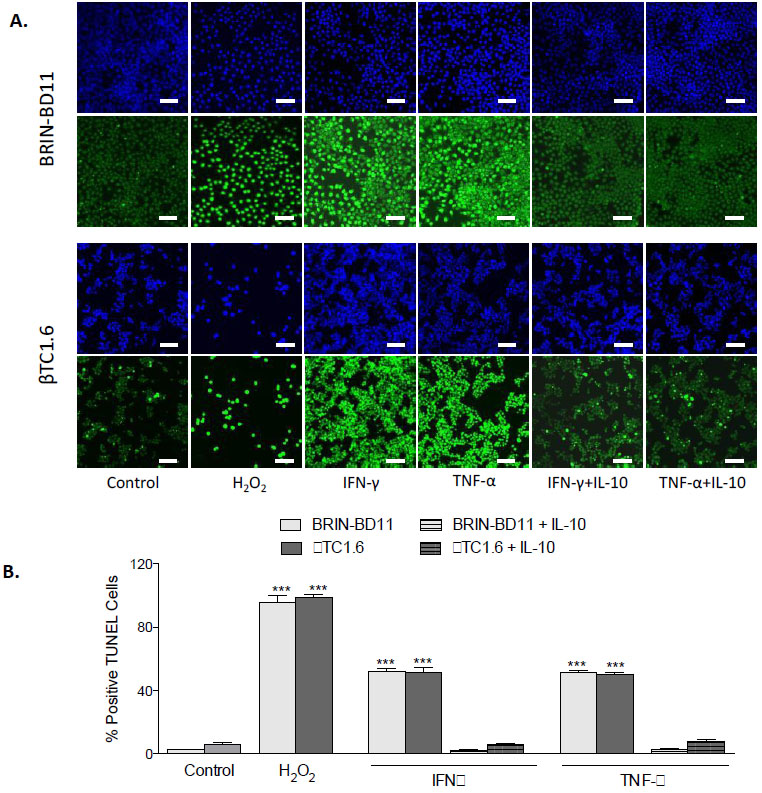
To confirm that observed reductions in cellular viability resulted from apoptosis rather than necrosis, the percentage of TUNEL positive cells was assessed following exposure to optimal concentrations of cytokines, in the presence and absence of MSC-CM (Fig. 2A). Exposure to 1 µg/ml IFN-γ caused an 8-fold increase in apoptosis in BRIN-BD11 cells (P<0.001) and a 28-fold increase in βTC1.6 cells (P<0.001). Treatment of BRIN-BD11 and βTC1.6 cells with 100 ng/ml TNF-α or 100 ng/ml IL-1β also elicited significant increases in the percentage of TUNEL positive cells (TNF-α: 7-fold increase in BRIN-BD11 cells and 26-fold increase in βTC1.6 cells (P<0.001); IL-1β: 6-fold increase in BRIN-BD11 cells and 21-fold increase in βTC1.6 cells (P<0.001) (Fig. 2B). Positive control (1% H2O2) resulted in 12- and 39-fold increases in apoptosis (P<0.001) in BRIN-BD11 and βTC1.6 cells, respectively (Fig. 2B). In all instances, increases in apoptotic frequency in response to cytokine challenge were largely reversed by MSC-CM (Fig. 2). Representative images suggest that MSC-CM is also protective against cytokine-driven apoptosis in primary islets (Supplementary Fig. S2).
3.2. MSC-CM Restores Glucose-Stimulated Insulin Secretion by Enhancing Islet Cell Viability, but not Function
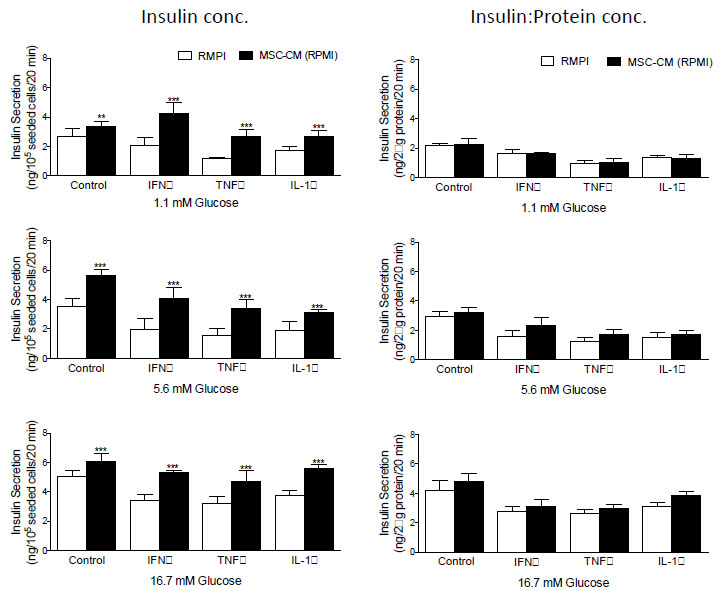
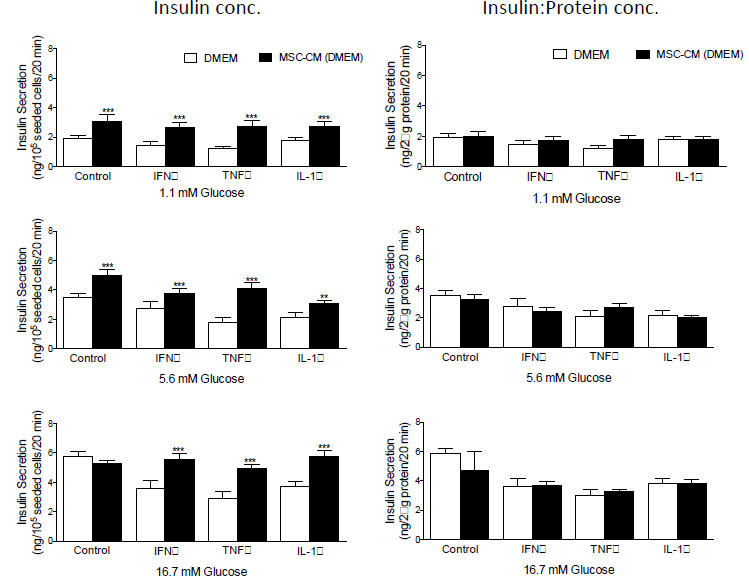
Examination of insulin secretion in response to 1.1, 5.6 and 16.7 mM D-glucose before and after the addition of MCS-CM revealed that in all instances, glucose-stimulated insulin secretion from BRIN-BD11 (Fig. 3) and βTC1.6 (Fig. 4) cells was significantly higher (P<0.01-P<0.001) in the presence of MSC-CM. At stimulatory concentrations of glucose (16.7 mM), a 1.2-fold increase (P<0.05) in insulin release was observed in untreated BRIN-BD11 cells cultured in the presence of MSC-CM (Fig. 3). A significant impact of MSC-CM on insulin release was not observed in untreated βTC1.6 cells (Fig. 4). However, MSC-CM resulted in significant enhancements in insulin release in cells treated with 1 µg/ml IFN-γ, 100 ng/ml TNF-α or 100 ng/ml IL-1β for 24h prior to acute exposure to glucose. This observation was true of all glucose concentrations tested and consistent for both the BRIN-BD11 (Fig. 3) and βTC1.6 (Fig. 4) cell lines. Culture of BRIN-BD11 cells in the presence of MSC-CM and 1 µg/ml IFN-γ, 100 ng/ml TNF-α or 100 ng/ml IL-1β for 24h prior to exposure to 16.7 mM glucose resulted in 1.5-fold increases in insulin release in all instances (P<0.001; Fig. 3). In the βTC1.6 cell line, MSC-CM resulted in 1.5-fold increases in insulin release in response to 16.7 mM glucose in cells treated with IFN-γ or IL-1β (P<0.001) and a 1.7-fold increase in cells treated with TNF-α (P<0.001, Fig. 4).
With the demonstration that MSC-CM protects against beta cell apoptosis, we wished to determine if the observed increase in insulin secretion was a direct consequence of enhanced beta cell survival. Therefore, data were standardized according to protein concentration, which acted as a surrogate for cell number in this instance. Following standardization, many of the apparent increases in insulin secretion were abolished indicating that improvements in glucose-stimulated insulin secretion likely result from enhancements in the viability of the cells, rather than augmentation of beta cell function (Fig. 3, BRIN-BD11 cells and Fig. 4, βTC1.6).
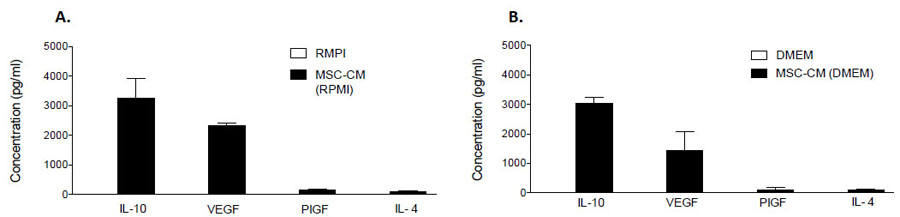
3.3. MSCs Secrete High Concentrations of Anti-Inflammatory Proteins
To explore the content of our MSC-CM, possible candidates were chosen based on data obtained from a secretome cytokine array (Data not shown). MSC-CM was analyzed for IL-4, IL-10, VEGF and PIGF content by commercially available ELISA assays. IL-10 was the most abundant of the candidates and measured at respective concentrations of 3270 ± 378 pg/ml and 3039 ± 122 pg/ml in RMPI1640 and DMEM MSC-CM, respectively (Fig. 5). Significant concentrations of VEGF (RPMI1640: 2315 ± 61 pg/ml; DMEM: 1423 ± 382 pg/ml), PIGF (RPMI1640: 153 ± 20 pg/ml; DMEM: 109± 46 pg/ml) and IL-4 (RPMI1640: 93 ± 7 pg/ml; DMEM: 107 ± 6 pg/ml) were also detected (Fig. 5). For comparison, the expression of each candidate protein was also assessed in RMPI1640 or DMEM medium that had not undergone conditioning by MSCs. These proteins were not detected in either medium, indicating that they are secreted products of hMSCs (Fig. 5).
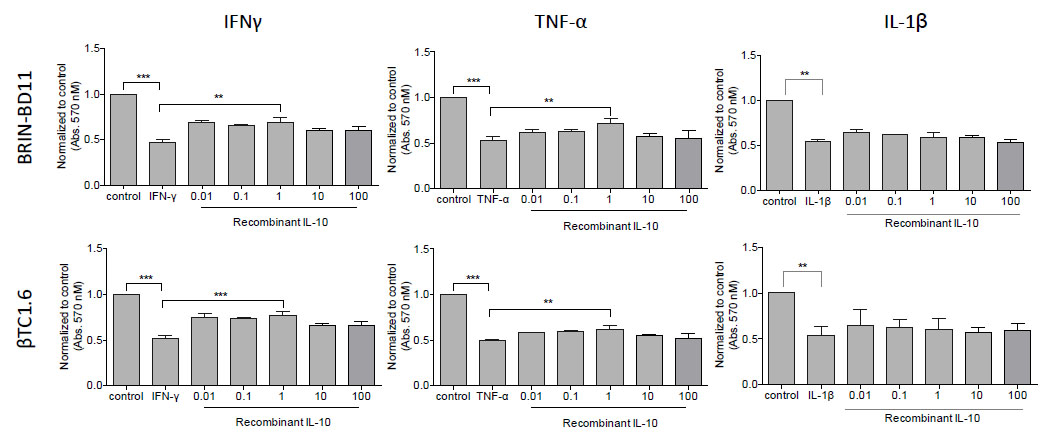
3.4. IL-10 Confers Protection Against IFN-γ or TNF-α-Induced Apoptosis
Then we assessed the impact of each of these candidates on cellular viability and apoptosis. BRIN-BD11 and βTC1.6 cells were exposed to rising concentrations (0.01 – 100 ng/ml) of recombinant IL-4, IL-10, VEGF and PIGF in the presence or absence of pro-apoptotic IFN-γ, TNF-α and IL-1β. A significant increase in cell viability was noted between the cells treated with IFN-γ or TNF-α alone and those treated with IFN-γ or TNF-α in the presence of recombinant IL-10. At 1 ng/ml IL-10, BRIN-BD11 cells showed a 46% improvement (P<0.001) in IFN-γ-driven reductions in cellular viability and a 25% improvement (P<0.01) in response to TNF-α treatment. Similar findings were observed in βTC1.6 cell lines (Fig. 6). IL-10 appeared to have no effect on IL-1β mediated reductions in cellular viability. Furthermore, the addition of IL-4, VEGF or PIGF conferred little protection against cytokine-driven reductions in cellular viability (Data not shown).
Next, we confirmed that improvements in cellular viability in response to IL-10 resulted from reductions in apoptosis. BRIN-BD11 and βTC1.6 cells were exposed to IFN-γ or TNF-α in the presence or absence of 1 ng/ml IL-10 and induction of apoptosis investigated by TUNEL assay. As shown in Fig. (7), significant (P<0.001) reductions in the number of TUNEL positive cells were observed in the presence of IL-10. In the BRIN-BD11 cell line, 95% reductions in the number of TUNEL positive cells were observed in the cells treated with IFN-γ and TNF-α in the presence of IL-10, compared with those treated with IFN-γ or TNF-α alone (Fig. 7B, P<0.001). In the βTC1.6, the number of TUNEL positive cells observed in response to IFN-γ and TNF-α was reduced by 88% and 84% respectively when IL-10 was present (Fig. 7B, P<0.001).
3.5. The Anti-Apoptotic Action of MSC-CM is Largely Driven by IL-10
We evaluated the expression of IL-10 receptors in BRIN-BD11 and βTC1.6 cells at the transcriptional level by RT-PCR. IL-10RA and IL-10RB mRNA transcription was confirmed in both BRIN-BD11 and βTC1.6 cells as shown in Figure S4 (Supplement). To determine if the anti-apoptotic actions of MSC-CM were partly facilitated by the anti-inflammatory action of IL-10, MSC-CM was depleted of IL-10 through the addition of 100 ng/ml of anti-IL-10 antibody (PeproTech). IL-10 depleted MSC-CM was applied to BRIN-BD11 and βTC1.6 cells along with 1 μg/ml IFN-γ or 100 ng/ml TNF-α. Cell viability was significantly reduced in the cells treated with IL-10 depleted MSC-CM (Fig. 8). In comparison with the cells treated with IFN-γ and MSC-CM, BRIN-BD11 cells displayed a 31 ± 1.4% reduction in cell viability when the cells were treated with IFN-γ plus IL-10-depleted MSC-CM. Under the same conditions, βTC1.6 cells displayed a 32 ± 3.7% reduction in viability (Fig. 8). Treatment of BRIN-BD11 and βTC1.6 cells with TNF-α plus IL-10 depleted MSC-CM resulted in respective 33 ± 3.2% and 30 ± 4.1% reductions in cell viability when compared with the cells cultured in the presence of TNF-α plus complete MSC-CM (Fig. 8). Furthermore, an almost complete reversal of the anti-apoptotic effect of MSC-CM was observed in the presence of the anti-IL-10 antibody (Fig. 9).
4. DISCUSSION
The immunomodulatory effects of MSCs are well established. Although the mechanism remains unclear, several studies have shown that MSC transplantation can improve the metabolic profile of diabetic animal models [15]. Some studies have suggested a cardinal role for the secretome and the paracrine signals it exerts (rather than stem cell differentiation) in the regenerative effects observed following therapeutic stem cell administration [16]. It is thought that the secretome consists of a complex set of proteins, growth factors, cytokines, angiogenic factors, hormones and extracellular matrix proteins [17], which have important biological roles including replication, cell growth, differentiation, and apoptosis [18]. In addition to the direct secretion of soluble factors, there is also increasing attention focused on the release of extracellular vesicles (exosomes, microvesicles) carrying potentially therapeutic, bioactive cargo [19-22]. Several studies have investigated the manner by which these soluble factors act and it is generally thought that they may either act directly, by mediating intracellular pathways in injured cells, or indirectly, by inducing the secretion of functionally active products from adjacent tissues [19].
In the present study, we sought to evaluate the anti-apoptotic effect of the MSC secretome. Conditioned medium from human bone marrow derived MSCs (MSC-CM) was added to insulin-secreting cell lines and primary islets in the presence or absence of cytokines known to promote beta cell apoptosis, namely IFN-γ, TNF-α, and IL-1β a synergistic effect of these cytokines on the demise of the beta cell [23]. However, the concentrations and interaction of pro-inflammatory cytokines are thought to vary significantly during the development of T1DM. This may explain the different levels of protection achieved through blocking the action of these cytokines in rat models of autoimmune diabetes [24]. The current study sought to understand the individual contribution of each cytokine in beta cell apoptosis and to identify specific mechanisms by which MSC-CM may confer protection against this process. Therefore, the cells were exposed to individual cytokines and their independent effects studied.
Unsurprisingly, IFN-γ TNF-α and IL-1β each induced apoptosis in beta cell models and primary islets. The addition of MSC-CM to cell lines and primary islets largely reversed cytokine-driven apoptosis. This is consistent with prior observations that MSCs and their secretome confer protection against cytokine-driven islet loss and that islet function is enhanced islet function through secreted products [25]. Here, MSC-CM restored glucose-stimulated insulin secretion in cytokine-treated cell lines. However, it has been shown that enhancements in insulin secretion in response to MSC co-culture likely result from improvements in cell viability rather than enhancements in the secretory function of the cells [26]. Our insulin secretory data was therefore standardized according to the protein content of the cells. The enhancements in glucose stimulated insulin secretion observed in the presence of MSC-CM were lost upon standardization of the data, suggesting that this effect was indeed linked to improvements in cellular viability rather than any direct enhancement in functionality of the cells.
Soluble factors secreted by MSCs are believed to alter the secretion profile of dendritic cells leading to increased production of anti-inflammatory cytokines like IL-10 and decreased production of inflammatory cytokines like IFN-γ and TNF-α [11]. MSCs can reduce T cell infiltration into pancreatic islets and the progression to diabetes through the induction of IL10-secreting FOXP3(+) T cells [27]. MSCs produce anti-apoptotic effects not only through their ability to restore the local microenvironment, but also by specifically producing anti-inflammatory or anti-apoptotic proteins including IL-10 [28]. Tang and colleagues also reported that MSC-treated cardiac cells displayed lower levels of pro-apoptotic factors including Bax and cleaved caspase 3 while the levels of pro-angiogenic factors including VEGF and FGF, were increased [29]. Consistently, the restoration of cardiac function in response to MSC therapy has been linked to the secretion of paracrine protective factors rather than myocardial regeneration [30].
In the current study, characterization of MSC-CM revealed significant concentrations of IL-4, IL-10, PIGF and VEGF. Prior work has shown that IL-4 and IL-10 can directly impact beta cell function and promote beta cell viability. Furthermore, circulating levels of both cytokines are reduced in T1D [31-33]. The potential cytoprotective mechanisms of IL-4 and IL-10 in beta cells are complex. As summarized by Russell and Morgan [34], both cytokines are thought to reduce oxidative stress and to inhibit various inflammatory pathways, including NF-κB, likely through stabilization of IκB [34]. The potential roles of PIGF and VEGF in beta cell survival are less well studied. PIGF is a member of the VEGF sub-family with confirmed roles in angiogenesis and vascular regeneration. PIGF-overexpressing transgenic mice displayed inflammation and evidence of metabolic disease when receiving a high-fat diet [35]. It has been suggested that VEGF governs the formation of intra-islet capillaries during embryogenesis [36] and improves graft revascularization when islets are implanted [37]. However, of the four abundant candidates identified in our MSC-CM, only IL-10 was found to prevent cytokine-driven apoptosis. Importantly, the anti-apoptotic effect of IL-10 was only observed in response to IFN-γ, or TNF-α challenge and not in response to IL-1β suggesting that the pathways involved are highly specific. IL-10 signals via unique receptor complexes that do not share homology with IL-4, PIGF, or VEGF signaling. Expression of both isoforms of the IL-10 receptor (IL-10RA and IL-10RB) was confirmed in the two cell lines used in this study (Supplementary Fig. S3), which is consistent with findings in human islets [34]. Indeed, the inhibition of IL-10 action by the addition of blocking antibody reversed the anti-apoptotic effects of MSC-CM. Overall, the protective effects of MSC-CM on islet cell survival appear to be largely IL-10-dependent.
CONCLUSION
In this study, we show that (1) factors secreted from MSCs are sufficient to promote islet beta cell survival in response to cytokine challenge, (2) this increase in survival is able to sustain glucose-stimulated insulin secretion in the face of inflammatory challenge and (3) IL-10 plays a significant part in the anti-apoptotic effects of MSC-CM, which may indicate some of the mechanisms by which MSCs confer protection on pancreatic islet beta cells.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
HUMAN AND ANIMAL RIGHTS
Not applicable.
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
The data that support the findings of this study are available from the corresponding author, [N. R. F.], upon reasonable request.
FUNDING
This work was supported by the Iraqi Ministry of Higher Education and Scientific Research (MOSHER) with grant no. S912, the European Union Regional Development Fund (ERDF) EU Sustainable Competitiveness Programme for N. Ireland; Northern Ireland Public Health Agency (HSC R&D) & Ulster University.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.